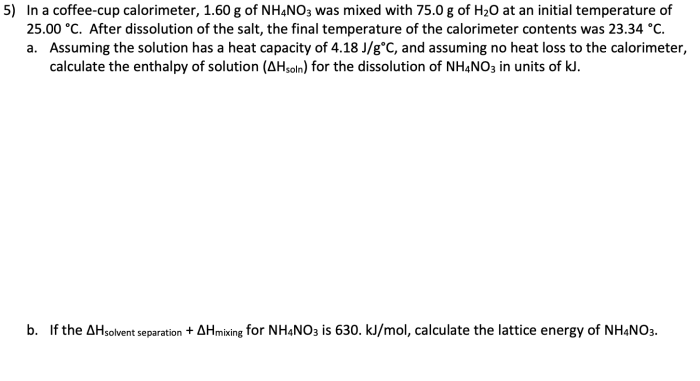
In a coffee cup calorimeter 1.60 g nh4no3 – In a coffee cup calorimeter, we embark on a scientific journey to unravel the intricacies of enthalpy change of dissolution. This experiment, using 1.60 g of NH4NO3, will illuminate the fundamental concepts and practical applications of this phenomenon.
As we delve into the experimental setup, we’ll dissect the components of the calorimeter and their roles in accurately measuring temperature changes. The step-by-step procedure will guide us through preparing the solution, monitoring temperature variations, and calculating the enthalpy change.
Enthalpy Change of Dissolution
Enthalpy change of dissolution refers to the heat change that occurs when a solute dissolves in a solvent. It is a measure of the energy required or released during the dissolution process.
Factors affecting the enthalpy change of dissolution include the nature of the solute and solvent, the concentration of the solution, and the temperature.
Experimental Setup
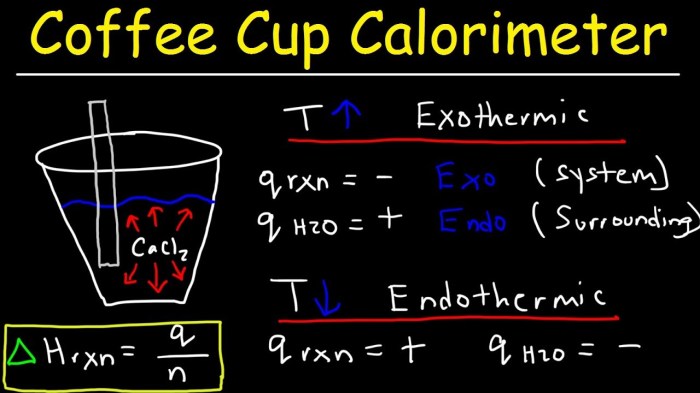
A coffee cup calorimeter is a simple and inexpensive device used to measure the enthalpy change of dissolution.
It consists of a Styrofoam cup, a thermometer, a stirrer, and a solution of known mass and temperature.
Procedure: In A Coffee Cup Calorimeter 1.60 G Nh4no3
- Prepare a solution of known mass and temperature.
- Measure the initial temperature of the solution.
- Add the solute to the solution and stir.
- Record the highest temperature reached by the solution.
- Calculate the enthalpy change of dissolution using the following formula:
- ΔH is the enthalpy change of dissolution
- m₁ is the mass of the solution
- C₁ is the specific heat capacity of the solution
- m₂ is the mass of the solute
- C₂ is the specific heat capacity of the solute
- ΔT is the change in temperature
ΔH = (m₁C₁ + m₂C₂)ΔT
Data Analysis

The data obtained from the experiment can be used to determine the enthalpy change of dissolution.
The enthalpy change is calculated using the formula provided in the Procedure section.
Applications
Enthalpy change of dissolution has applications in various fields, including:
- Chemistry: To determine the solubility and stability of compounds.
- Biology: To study the interactions between biomolecules.
- Pharmacology: To design drugs with specific solubility and absorption properties.
Detailed FAQs
What is enthalpy change of dissolution?
Enthalpy change of dissolution is the heat absorbed or released when a substance dissolves in a solvent.
What factors affect the enthalpy change of dissolution?
Factors affecting enthalpy change of dissolution include the nature of the solute and solvent, their concentrations, and the temperature.
What is the purpose of a coffee cup calorimeter?
A coffee cup calorimeter is a simple and inexpensive device used to measure enthalpy changes in reactions that occur in solution.