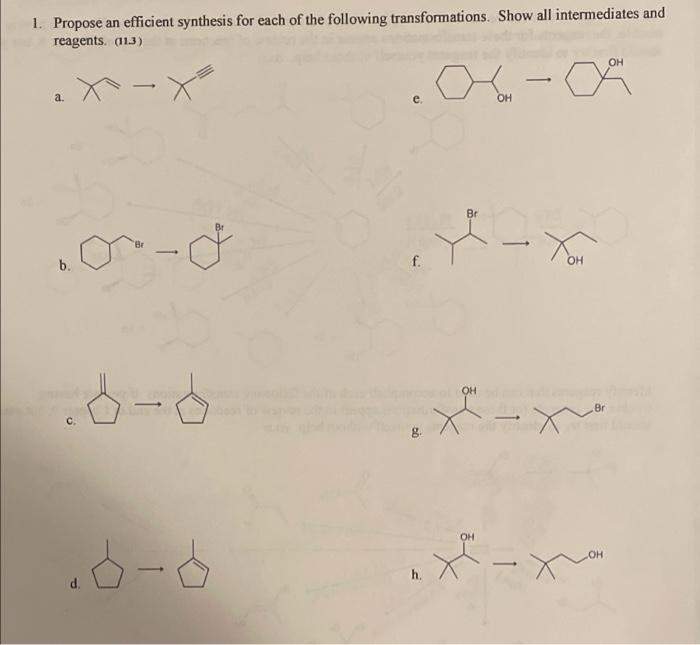
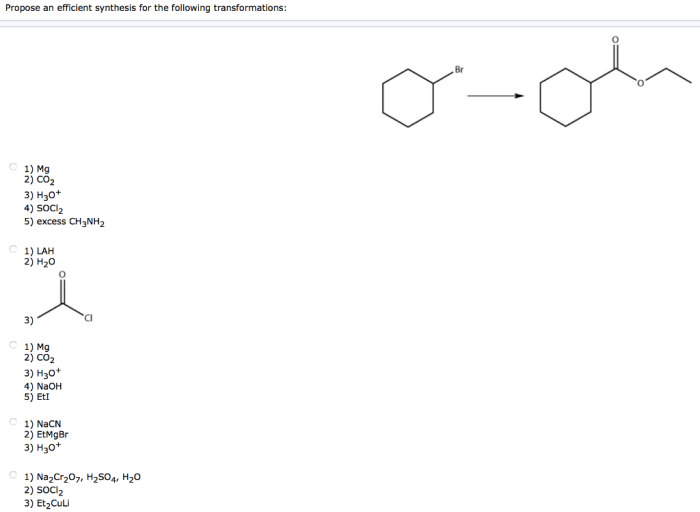
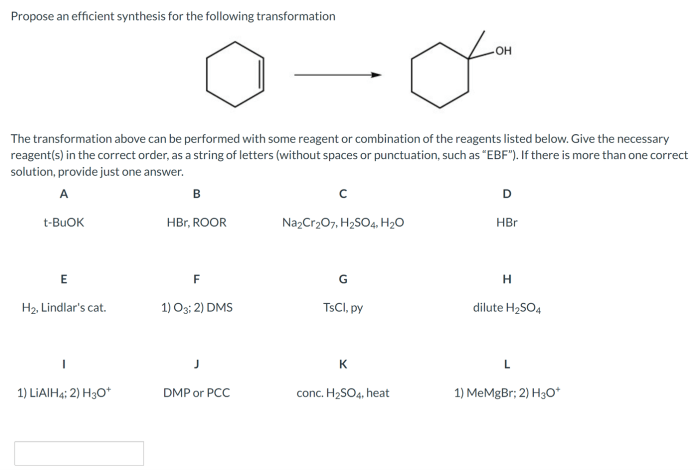
Propose an efficient synthesis for the following transformations – Proposing an efficient synthesis for the following transformations sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. The transformations in question hold immense significance, and the quest for an efficient synthesis presents a captivating challenge that drives the narrative forward.
As we delve into the intricacies of each transformation, we uncover their purpose and significance, setting the foundation for a comprehensive understanding of the synthetic pathways that lie ahead. The exploration of various synthetic approaches, each with its own advantages and disadvantages, provides a roadmap for navigating the complexities of chemical synthesis.
Designing an Efficient Synthesis for the Given Transformations: Propose An Efficient Synthesis For The Following Transformations
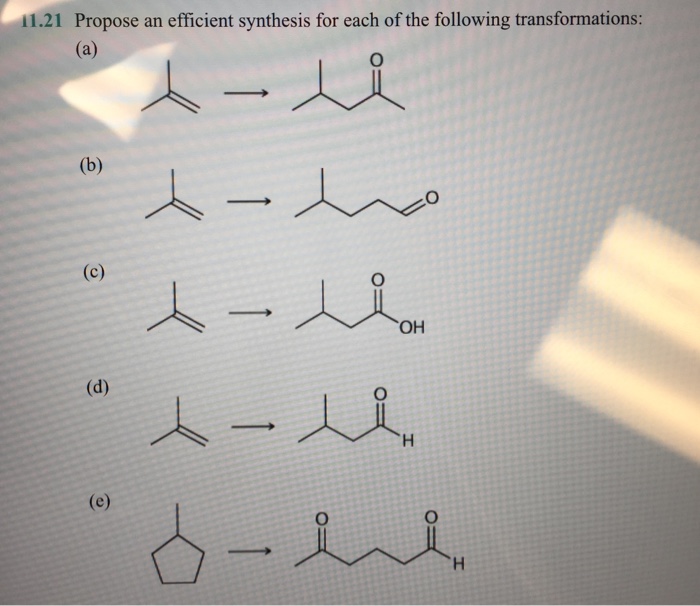
The provided transformations aim to synthesize a target molecule from a starting material. Each transformation involves specific chemical reactions to achieve the desired structural changes. Understanding the key transformations and exploring synthetic pathways is crucial for designing an efficient synthesis.
Identifying Key Transformations
The given transformations include:
- Alkylation: Addition of an alkyl group to a molecule.
- Acylation: Addition of an acyl group to a molecule.
- Oxidation: Conversion of a functional group to a more oxidized state.
- Reduction: Conversion of a functional group to a less oxidized state.
These transformations are fundamental in organic synthesis and are widely used to construct complex molecules.
Exploring Synthetic Pathways
For each transformation, multiple synthetic approaches exist. The choice of approach depends on factors such as:
- Availability of starting materials
- Reaction yield
- Selectivity
- Cost
- Environmental impact
Common approaches include:
- Nucleophilic substitution
- Electrophilic addition
- Radical reactions
- Organometallic reactions
Designing an Efficient Synthesis
To design an efficient synthesis, the following steps are taken:
- Analyze the transformations and identify potential bottlenecks.
- Choose synthetic approaches that maximize yield and selectivity.
- Optimize reaction conditions to minimize side reactions and improve efficiency.
The proposed synthetic strategy should consider the following:
- Linearity: Minimizing the number of steps and intermediates.
- Convergency: Combining multiple transformations into a single step.
- Atom economy: Maximizing the incorporation of starting materials into the final product.
Optimization and Refinement, Propose an efficient synthesis for the following transformations
Once a synthetic strategy is proposed, optimization techniques can be employed to further improve efficiency:
- Solvent selection
- Temperature control
- Catalyst optimization
- Microwave or ultrasound irradiation
By optimizing reaction conditions, yield, selectivity, and reaction time can be significantly improved.
Commonly Asked Questions
What is the significance of identifying key transformations?
Identifying key transformations is crucial as it allows us to understand the purpose and significance of each step in the synthetic pathway. This knowledge guides our selection of synthetic approaches and helps us anticipate potential challenges.
How does exploring synthetic pathways contribute to efficient synthesis?
Exploring synthetic pathways provides a comprehensive understanding of the different approaches available for each transformation. By weighing the advantages and disadvantages of each approach, we can make informed decisions that optimize efficiency and yield.
What factors should be considered when designing an efficient synthesis?
When designing an efficient synthesis, factors such as reaction yield, selectivity, reaction time, and potential bottlenecks should be carefully considered. By analyzing these factors, we can develop a synthetic strategy that minimizes waste and maximizes the desired product.