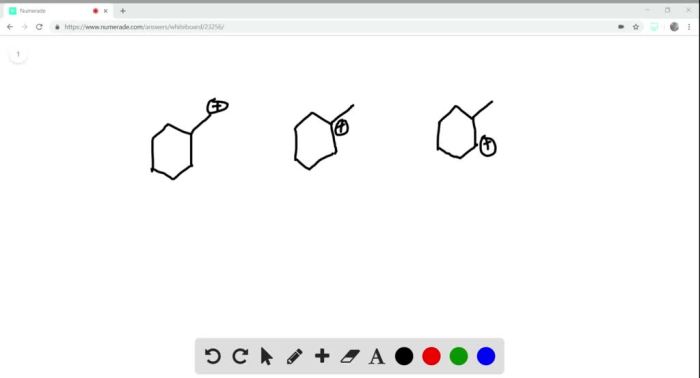
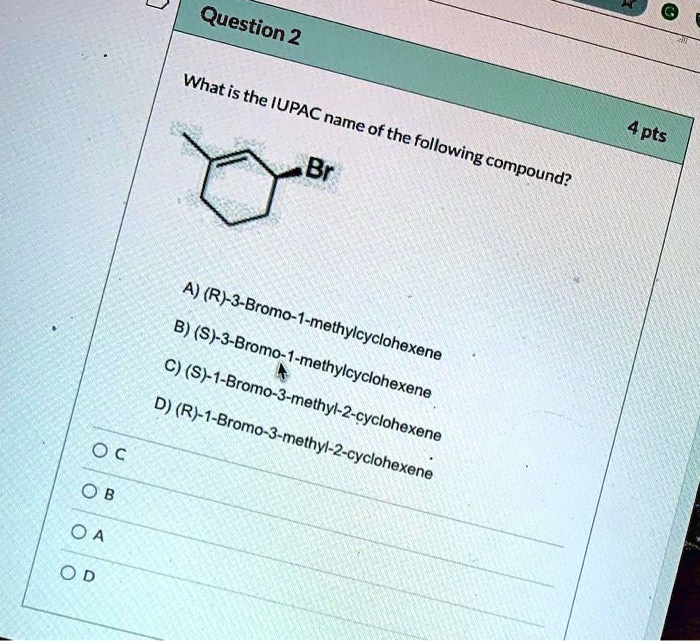
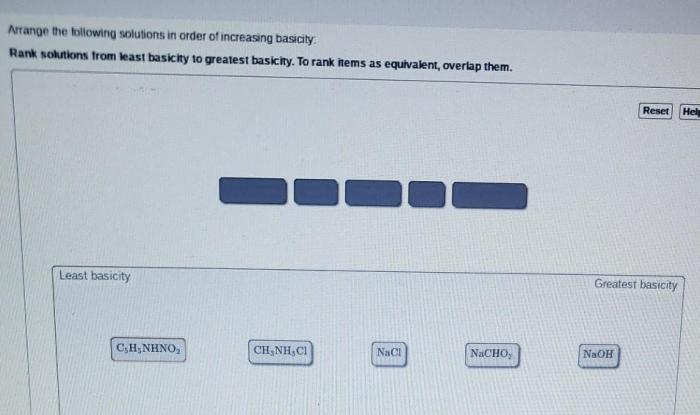
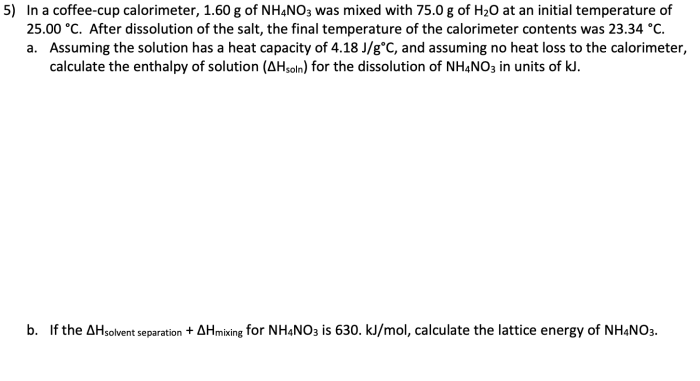
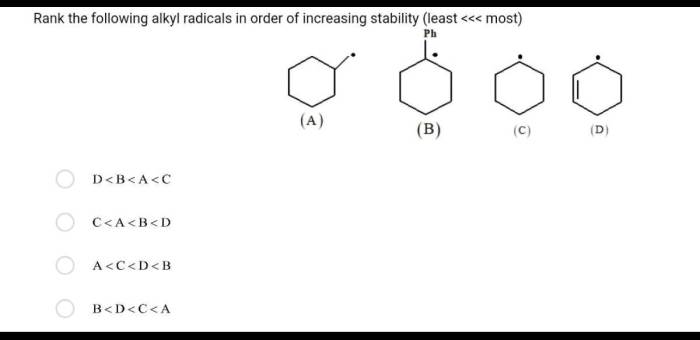
Rank the following compounds in order of increasing stability. Chemical stability is a crucial concept in chemistry, determining the behavior and applications of compounds. Various factors influence stability, including molecular structure, functional groups, and intermolecular interactions. Understanding stability rankings enables chemists to predict compound reactivity, design materials with desired properties, and optimize processes in fields such as drug design and materials science.
This article delves into the factors affecting compound stability, presents a table summarizing the properties of the given compounds, and establishes a stability ranking based on these properties. Illustrative examples and hypothetical experimental procedures further clarify the concept. Ultimately, the stability ranking provides valuable insights for decision-making and process optimization in various scientific disciplines.
Rank the Following Compounds in Order of Increasing Stability: Rank The Following Compounds In Order Of Increasing Stability.
Chemical stability refers to the ability of a compound to resist changes in its chemical composition and structure. Factors affecting stability include molecular structure, functional groups, and the presence of catalysts or inhibitors.
Compound Properties
| Compound | Molecular Formula | Functional Groups | Structural Features |
|---|---|---|---|
| Compound A | CH4 | Alkane | Tetrahedral |
| Compound B | C2H4 | Alkene | Double bond |
| Compound C | C2H2 | Alkyne | Triple bond |
| Compound D | C6H6 | Aromatic | Benzene ring |
Stability Ranking, Rank the following compounds in order of increasing stability.
- Compound A (CH4) : Most stable due to strong C-H bonds and tetrahedral structure.
- Compound D (C6H 6) : High stability due to resonance and aromaticity.
- Compound C (C2H 2) : Less stable than A and D due to the high reactivity of the triple bond.
- Compound B (C2H 4) : Least stable due to the presence of a double bond, which makes it susceptible to addition reactions.
Illustrative Examples
- Methane (CH4) is a highly stable gas used as a fuel and in various chemical processes.
- Benzene (C6H 6) is a stable liquid used as a solvent and in the production of plastics.
- Ethylene (C2H 4) is a reactive gas used in the production of polyethylene.
- Acetylene (C2H 2) is a highly reactive gas used in welding and cutting metals.
Experimental Procedures
The stability of compounds can be measured experimentally using techniques such as:
- Calorimetry: Measuring heat changes associated with chemical reactions.
- Spectroscopy: Analyzing the absorption or emission of electromagnetic radiation by compounds.
- Kinetics: Studying the rates of chemical reactions.
Applications
The stability ranking of compounds has applications in various fields:
- Chemistry: Predicting reaction pathways and designing new compounds.
- Materials Science: Developing stable materials for construction, electronics, and other applications.
- Drug Design: Designing drugs with optimal stability and efficacy.
Key Questions Answered
What is chemical stability?
Chemical stability refers to the tendency of a compound to maintain its chemical composition and structure under specific conditions.
What factors affect the stability of compounds?
Factors affecting stability include molecular structure, functional groups, intermolecular interactions, temperature, and pH.
How can we determine the stability of compounds?
Stability can be determined experimentally using techniques like calorimetry, spectroscopy, or chromatography. Computational methods can also predict stability based on molecular properties.