Arrange the elements in decreasing order of first ionization energy. This topic delves into the fascinating world of ionization energy, a fundamental concept in chemistry that governs the behavior of atoms and molecules. Join us on an enlightening journey as we explore the factors influencing ionization energy, its periodic trends, and its practical applications.
Delving deeper into the intricacies of ionization energy, we will uncover the reasons behind its variation across the periodic table, unraveling the interplay between atomic structure and the energy required to remove an electron. By examining specific elements and their ionization energies, we will gain insights into the underlying principles that shape the chemical properties of matter.
Understanding Ionization Energy: Arrange The Elements In Decreasing Order Of First Ionization Energy
Ionization energy refers to the energy required to remove an electron from an atom or ion in its gaseous state. It is a crucial concept in chemistry as it provides insights into the stability and reactivity of elements.
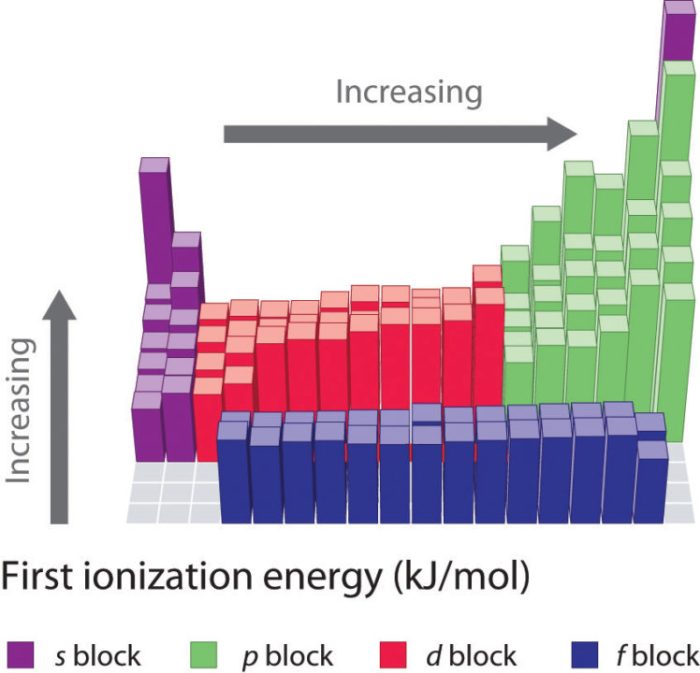
Factors affecting ionization energy include atomic size, nuclear charge, and electron configuration.
Trends in Ionization Energy
Across Periods
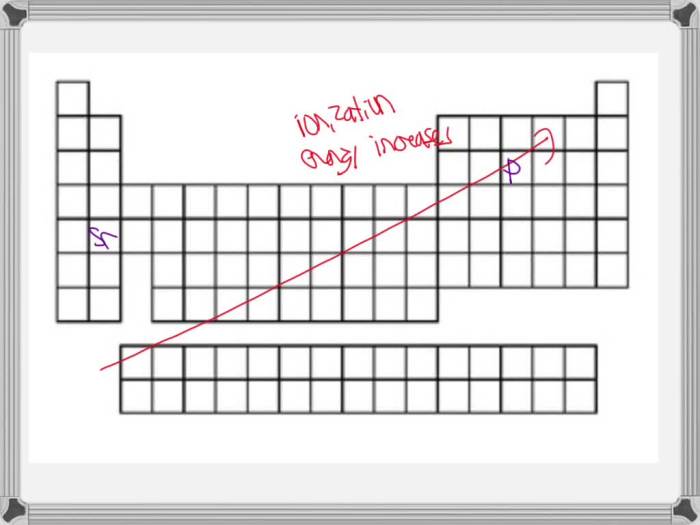
Ionization energy generally increases from left to right across a period in the periodic table. This is because the effective nuclear charge experienced by the outermost electrons increases, making it more difficult to remove them.
Across Groups, Arrange the elements in decreasing order of first ionization energy
Ionization energy generally decreases down a group in the periodic table. This is because the outermost electrons are located further from the nucleus, experiencing a weaker attractive force.
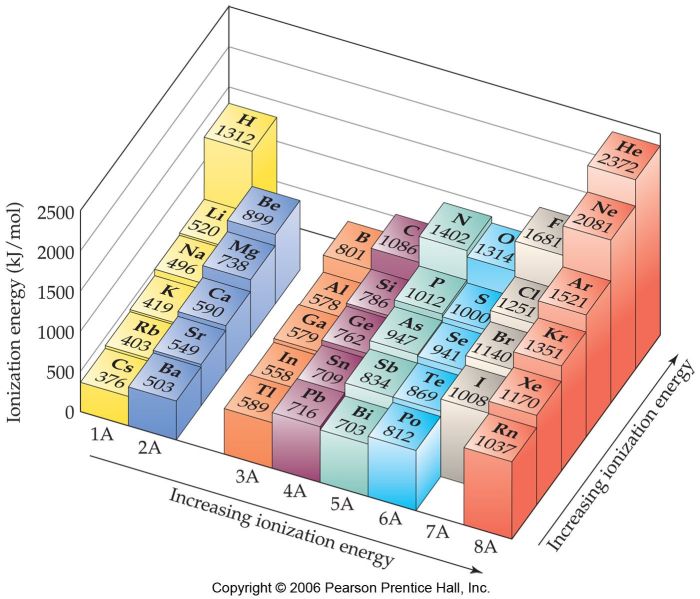
Periodic Table and Ionization Energy
| Element Symbol | Atomic Number | First Ionization Energy (eV) |
|---|---|---|
| He | 2 | 24.6 |
| Ne | 10 | 21.6 |
| Ar | 18 | 15.8 |
| Kr | 36 | 14.0 |
| Xe | 54 | 12.1 |
| Rn | 86 | 10.7 |
Exceptions and Explanations
Exceptions to the general trends in ionization energy occur due to factors such as:
- Electron configuration: Elements with half-filled or completely filled orbitals exhibit higher ionization energies.
- Lanthanide contraction: The gradual decrease in atomic radii of lanthanide elements leads to increased ionization energies.
Applications of Ionization Energy Data
Ionization energy data finds applications in various fields:
- Predicting chemical reactivity
- Designing new materials
- Understanding astrophysical phenomena
- Developing plasma technologies
Q&A
What is ionization energy?
Ionization energy is the energy required to remove an electron from an atom or ion in its gaseous state.
Why does ionization energy vary across the periodic table?
Ionization energy generally increases from left to right across a period and decreases from top to bottom within a group. This is due to changes in atomic radius and nuclear charge.
What are some applications of ionization energy data?
Ionization energy data is used in various fields, including predicting chemical reactivity, understanding plasma behavior, and designing materials with specific properties.