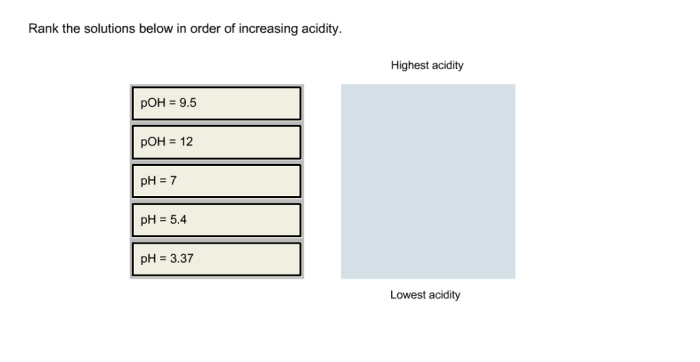
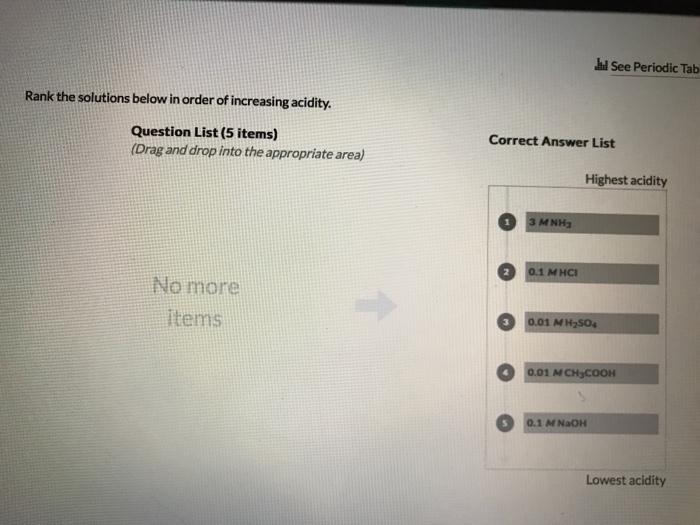
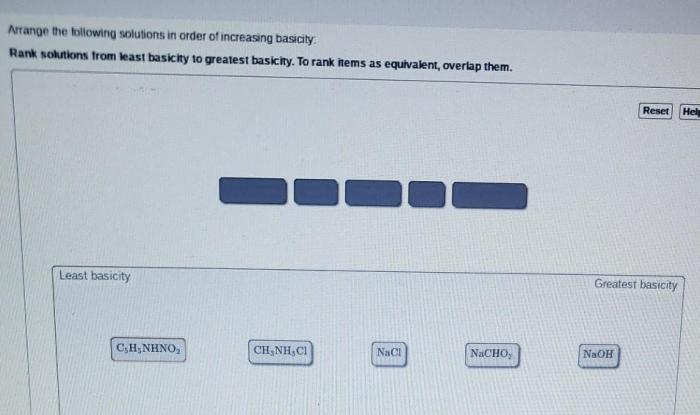
Rank the solutions below in order of increasing acidity – In the realm of chemistry, understanding the concept of acidity is paramount. This guide delves into the intricacies of acidity, exploring the factors that influence the strength of solutions and their corresponding pH values. By unraveling the mysteries of acid-base equilibria, neutralization reactions, and solvent effects, we empower you to rank solutions in order of increasing acidity, a skill with far-reaching applications in diverse fields.
Acidic Strength
Acidity is a measure of the concentration of hydrogen ions (H+) in a solution. The pH scale is used to quantify acidity, with lower pH values indicating higher acidity. Strong acids have a high concentration of H+ ions and low pH values, while weak acids have a low concentration of H+ ions and high pH values.
Factors Influencing Acid Strength
- Bond strength:The strength of the bond between the hydrogen ion and the rest of the molecule. Stronger bonds result in weaker acids.
- Electronegativity:The ability of an atom to attract electrons. More electronegative atoms form stronger bonds with hydrogen, resulting in weaker acids.
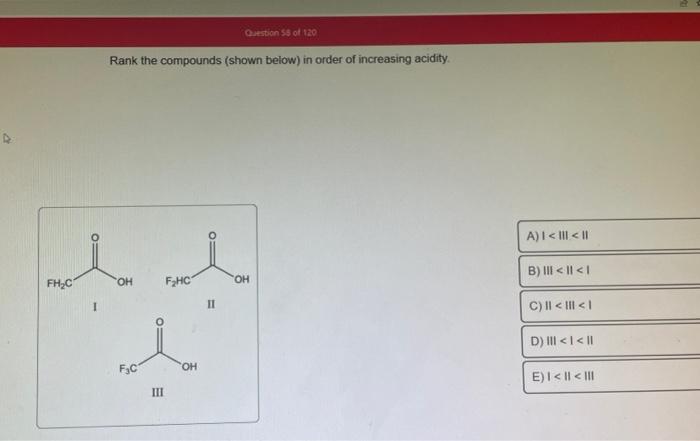
- Molecular structure:The arrangement of atoms within the molecule can affect the acidity. For example, carboxylic acids are more acidic than alcohols due to the presence of a carbonyl group.
Solution Concentration
The concentration of a solution is the amount of solute dissolved in a given amount of solvent. The concentration of an acid solution affects its acidity. Higher concentrations of acid result in lower pH values and higher acidity.
Methods for Measuring Concentration, Rank the solutions below in order of increasing acidity
- Titration:A method that involves adding a known concentration of base to an acid solution until the solution reaches a neutral pH.
- Conductivity:A method that measures the electrical conductivity of a solution, which is proportional to the concentration of ions.
- Spectrophotometry:A method that measures the absorbance of light by a solution, which can be used to determine the concentration of specific ions.
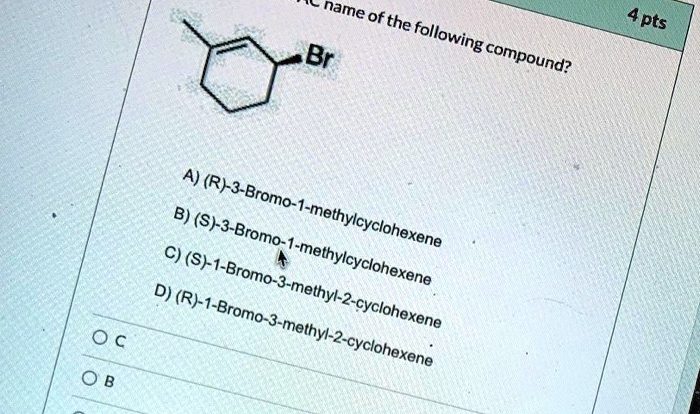
Chemical Structure: Rank The Solutions Below In Order Of Increasing Acidity
The chemical structure of an acid plays a crucial role in determining its acidity. Different functional groups have different acid-dissociation constants (Ka), which measure the strength of the acid.
Functional Groups and Acidity
- Carboxylic acids:Have a Ka of around 10^-5 and are relatively strong acids due to the presence of the carbonyl group.
- Alcohols:Have a Ka of around 10^-16 and are weak acids due to the weaker bond between the hydrogen and oxygen atoms.
- Phenols:Have a Ka of around 10^-10 and are stronger acids than alcohols due to the resonance stabilization of the phenoxide ion.
Temperature Effects
Temperature can affect the acidity of a solution. In general, increasing temperature increases the acidity of strong acids and decreases the acidity of weak acids.
Underlying Mechanisms
- Strong acids:The increase in temperature increases the kinetic energy of the molecules, making it easier for the hydrogen ions to dissociate from the acid.
- Weak acids:The increase in temperature favors the recombination of hydrogen ions and conjugate bases, decreasing the acidity.
Solvent Effects
The solvent in which an acid is dissolved can influence its acidity. Different solvents have different dielectric constants, which affect the ability of the solvent to solvate the ions.
Solvent Properties and Acidity
- Polar solvents:Have a high dielectric constant and solvate ions well, resulting in weaker acidity.
- Nonpolar solvents:Have a low dielectric constant and do not solvate ions well, resulting in stronger acidity.
Acid-Base Equilibria
Acid-base equilibria refer to the reversible reaction between an acid and a base, resulting in the formation of conjugate acid-base pairs. The equilibrium constant (Keq) for an acid-base reaction is a measure of the relative strengths of the acid and base.
Factors Influencing Equilibrium Position
- Initial concentrations:The initial concentrations of the acid and base affect the position of equilibrium.
- Temperature:Temperature can shift the equilibrium position towards the products or reactants.
- Solvent effects:The solvent can influence the equilibrium position by affecting the solvation of the ions.
Neutralization Reactions
Neutralization reactions are chemical reactions between an acid and a base that result in the formation of a salt and water. The pH of the resulting solution depends on the strengths of the acid and base.
Applications of Neutralization Reactions
- Titrations:Used to determine the concentration of an unknown acid or base.
- Buffer solutions:Used to maintain a specific pH range in a solution.
- Antacids:Used to neutralize stomach acid and relieve heartburn.
Applications of Acidity Ranking
Ranking solutions in order of increasing acidity is important in various fields, including:
Applications in Chemistry
- Acid-base titrations:To determine the equivalence point in a titration.
- Buffer solutions:To select the appropriate buffer for a given pH range.
- Organic synthesis:To control the acidity of reaction conditions.
Key Questions Answered
What factors influence the acidity of a solution?
The strength of an acid, solution concentration, chemical structure, temperature, and solvent properties all play a role in determining acidity.
How can I measure the acidity of a solution?
The pH scale is a widely used method for measuring acidity, with lower pH values indicating higher acidity.
What are the applications of ranking solutions by acidity?
Acidity ranking finds applications in fields such as chemistry, biology, medicine, and environmental science, where it aids in optimizing reactions, preserving biological systems, and assessing environmental impact.